Please note:
If your test report was generated for a patient in the state of New York or prior to December 10, 2024:
Please click here for more information about the Galleri Test
Supplemental information for the Galleri® test report
The Galleri test does not detect all cancers and should be used in addition to, and not instead of, routine cancer screening tests recommended by clinical guideline organizations such as the American Cancer Society or the US Preventive Services Task Force (USPSTF).
The Galleri test does not detect all cancers and should be used in addition to, and not instead of, routine cancer screening tests recommended by clinical guideline organizations such as the American Cancer Society or the US Preventive Services Task Force (USPSTF).
The Galleri test is supported by the PATHFINDER and CCGA Studies
In a 2024 analysis, overall specificity and sensitivity of Cancer Signal Detected were maintained and Cancer Signal Origin Prediction Accuracy was improved.3
About the PATHFINDER Study1
The PATHFINDER Study (Clinicaltrials.gov identifier: NCT04241796) is a prospective, multi-center, interventional, return of results study (N=6,662) to assess the implementation of an earlier version of the Galleri multi-cancer early detection (MCED) test in a clinical setting. Later, blood samples were reanalyzed with the current version of Galleri. The study included men and women ≥50 years of age without clinical suspicion of cancer at time of enrollment who were recruited into two cohorts.
The first cohort included participants with additional cancer risk (e.g., a history of smoking, prior cancer with treatment completed more than 3 years ago, or genetic cancer predisposition). The second cohort included participants without additional cancer risk. The patients were followed for 12 months.
The primary objective was to assess the extent and types of diagnostic testing required to achieve diagnostic resolution among individuals with a positive test. The secondary objectives were to evaluate test performance for cancer detection, and to assess participant-reported outcomes and perceptions about an MCED test.
A subset of PATHFINDER study including 1893 participants with no cancer diagnosis and completed 12 month follow-up was included in the 2024 clinical validation analysis of this test version.3
About the CCGA Study2
The Circulating Cancer Genome Atlas (CCGA) study (Clinicaltrials.gov identifier: NCT02889978) is a large, prospective, multicenter, case-control, observational clinical study with longitudinal follow-up which enrolled 15,254 participants (8,584 (56%) with a recent diagnosis of cancer and 6,670 (44%) without a recent diagnosis of cancer) from 141 clinical institutions and multiple networks throughout the United States, and one site in Canada, from August 2016 to February 2019. This study was divided into three sub-studies for the purpose of training and validation of the Galleri test. The third CCGA sub-study (CCGA3) validated performance of the Galleri test in 4,077 participants with confirmed status: 1,254 participants without cancer confirmed at one year follow-up and 2,823 cancer participants. Participants were men and women ≥ 20 years of age (approximately 80% over the age of 50 years), without prior history of cancer. Cancer participants had stage I (30.1%), stage II (24.9%), stage III (20.0%), stage IV (21.9%) cancer, or cancer that does not have stages (2.4%).
A subset of CCGA3 study including 944 cancer participants was included in the 2024 clinical validation analysis of this test version.3
Key primary objectives were to evaluate test performance for cancer signal detection (specificity, overall sensitivity, sensitivity by clinical stage) and signal origin prediction (accuracy).
Cancer types detected by a shared signal in the Galleri test in the CCGA3 Sub-study2,4

Sensitivity
Galleri can detect a shared cancer signal across more than 50 cancer types from a single blood draw.2,4 Sensitivity by tumor type and stage varies due to multiple host and tumor factors. For instance more aggressive cancers, such as pancreatic cancer, tend to release more cell-free DNA into the bloodstream at early stages and are more likely to be detected by the Galleri test.2,5


Other cancer types include: Adrenal (N = 1), Ampulla of Vater (N = 1), Brain (N = 6), Choriocarcinoma (N = 1), Mesothelioma (N = 7), Non-melanoma Non-BCC/SCC Skin Cancer (N = 2), Other/Unspecified (N = 10), Penis (N = 1), Small Intestine (N = 13), Testis (N = 6), Thymus (N = 2), Vagina (N = 2), Vulva (N = 7).

For multiple primaries, highest clinical stage was selected.
Cancer Signal Origin Prediction
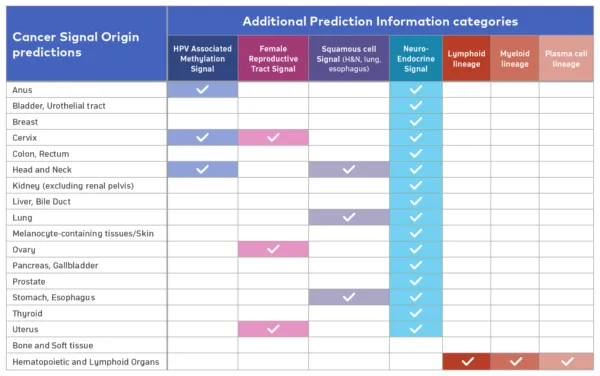
A Cancer Signal Origin (CSO) prediction will be provided for all Cancer Signal Detected results. There are 18 CSO predictions (see table below). In addition, certain cancers contain unique cancer biology patterns which can provide Additional Prediction Information to aid in the diagnostic evaluation. In approximately 40% of Cancer Signal Detected results, there may be one of seven Additional Prediction Information categories (see table below). Each Cancer Signal Detected result will have a Cancer Signal Origin prediction only OR a Cancer Signal Origin prediction and a single Additional Prediction Information.3
Check marks indicate Additional Prediction information that may accompany the CSO. A Cancer Signal Detected result will report a Cancer Signal Origin prediction OR a Cancer Signal origin prediction and a single category of Additional Prediction Information.
Have questions about the Galleri test report?
Please contact GRAIL Customer Service and ask to speak with a Medical Information Specialist.
- Galleri is a screening test and does not diagnose cancer. Diagnostic testing is needed to confirm cancer.
- The Galleri test looks for a signal associated with cancer and does not predict future genetic risk for cancer.
- If a cancer signal is detected, Galleri can predict the tissue or organ associated with the signal to help healthcare providers determine next steps.
- The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood.
- False positive and false negative results do occur.
- Galleri should be used in addition to healthcare provider recommended screening tests.
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The Galleri test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. Galleri is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of Galleri is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g. imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False-positive (a cancer signal detected when cancer is not present) and false-negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed and its performance characteristics were determined by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes.
- Schrag D, McDonnell CH, Nadauld L, et al. A prospective study of a multi-cancer early detection blood test. Ann Oncol. 2022:33(supp 7):S9030. DOI:https://doi.org/10.1016/j.annonc.2022.07.1029.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-77. DOI:https://doi.org/10.1016/j.annonc.2021.05.806.
- GRAIL, Inc. Data on file: VV-TMF-59592 on clinical validation analysis in a subset of CCGA3 study including 944 cancer participants and a subset of PATHFINDER study including 1893 non-cancer participants
- Amin MB, et al. (Eds.). AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017.
- Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-759. DOI:https://doi.org/10.1016/j.annonc.2020.02.011.